Science
FDA to Phase Out Animal Testing Requirement for Some Drugs in “Win for Public Health & Ethics”
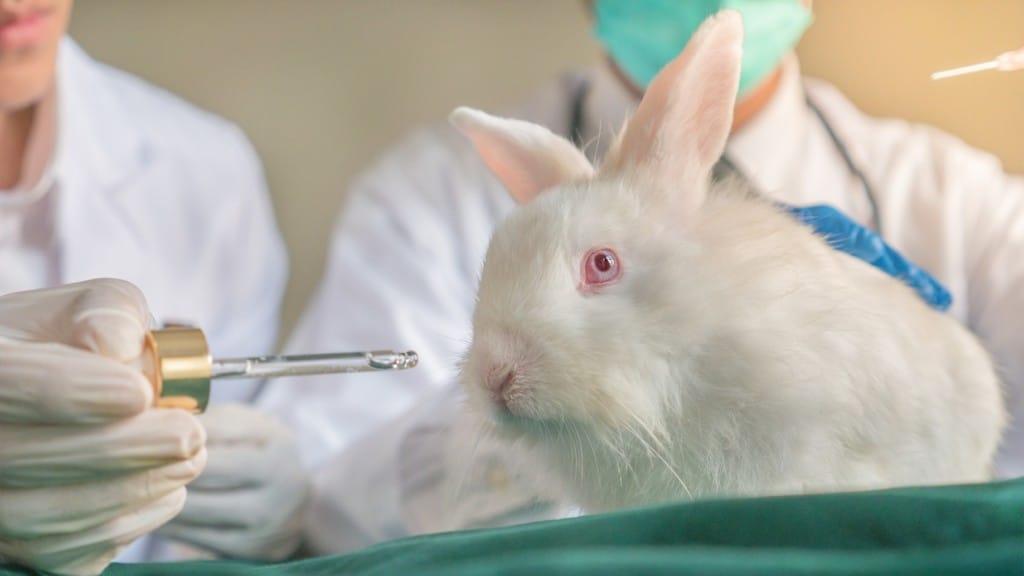
The FDA announced plans to phase out animal testing for monoclonal antibody therapies and other drugs, opting for more effective human-relevant methods such as AI-based computational models and lab testing with cell lines and organoids. This shift aims to improve drug safety, speed up evaluation processes, reduce animal experimentation, lower costs, and ultimately decrease drug prices. The FDA will update guidelines to consider data from these new methods, potentially streamlining reviews for companies with strong non-animal safety data.
The FDA will work with federal agencies to adopt modern drug testing methods and launch a pilot program allowing some developers to use primarily non-animal-based testing strategies. Commissioner Makary stated that this initiative marks a paradigm shift in drug evaluation, promising faster, more reliable treatments for Americans, while also reducing research costs, and drug prices. Leveraging AI, human organ models, and real-world data, this move is seen as a win-win for public health and ethics.
The FDA will work with federal agencies to adopt modern drug testing methods and launch a pilot program allowing some developers to use primarily non-animal-based testing strategies. Commissioner Makary stated that this initiative marks a paradigm shift in drug evaluation, promising faster, more reliable treatments for Americans, while also reducing research costs, and drug prices. Leveraging AI, human organ models, and real-world data, this move is seen as a win-win for public health and ethics.
*This summary was generated using AI.